This article was authored by Grace Saba, associate professor, and Josh Kohut, professor, faculty in Rutgers University’s Center for Ocean Observing Leadership (RUCOOL) in the Department of Marine and Coastal Sciences.
From late April to late September, Rutgers researchers used underwater robots, called gliders, to track ocean water quality along the New Jersey coast. Through a series of glider deployments, water quality measures of oxygen concentrations and pH were mapped from surface to bottom along the New Jersey coast.
From August through September, much of the bottom water sampled from Sandy Hook south to Tuckerton, and from nearshore (15 meter, or 50 feet, water depth) to deeper depths (60 meter, or 200 feet, water depth), exhibited dissolved oxygen concentrations less than 5 mg/liter and pH values less than 7.75.
Coast-wide, hypoxic levels of dissolved oxygen (concentrations < 3 mg/liter) were observed at shallower, more inshore locations. In addition to low pH measured in bottom waters, which is indicative of ocean acidification, aragonite saturation state (a relevant metric for biological impacts of ocean acidification) was calculated to be < 1 in several locations. Normal, more optimal levels in seawater typically include dissolved oxygen concentrations > 7 mg/liter, pH of 8.1, and aragonite saturation states > 3.
Saba and Kohut discuss the implications for their summer observations of low dissolved oxygen and pH off the coast of New Jersey, and offer some next steps in their research.
Why are these values concerning?
As is true for land animals, oxygen is essential to ocean animals. Dissolved oxygen concentrations at or below 5 mg/liter is considered problematic for marine life. Although concentrations between 3-5 mg/liter may not be low enough to directly cause death in many marine animals, research focused on New Jersey species has identified other negative impacts such as reduced metabolism, feeding, growth, and reproduction at these levels. Lower hypoxic concentrations of dissolved oxygen (< 3 mg/liter) have been directly associated with mortalities in some organisms in New Jersey and in other coastal regions around the world.
Increased carbon dioxide in seawater leads to a series of chemical reactions that increases the acidity of the ocean (measured as a reduction in pH) and reduces carbonate ions that are vital to the production of shells and other protective structures of marine animals (made of calcium carbonate, such as aragonite). As such, aragonite saturation state is used as an indicator of ocean acidification because as the ocean absorbs atmospheric carbon dioxide, both pH and aragonite saturation state decrease, and can lead to reduced survival, calcification rates, growth, and reproduction in marine animals. When aragonite saturation state is less than 1, shells and other calcium carbonate structures begin to dissolve, but some organisms become susceptible at levels below 3.
Any one stressor may not itself be an issue due to the resiliency of many coastal species to fluctuating natural environmental conditions. However, when more than one stressor occurs simultaneously, an organism may become unable to fully withstand changes. The impacts of multiple stressors occurring simultaneously on organism health is much less well known. The co-occurrence of low dissolved oxygen and pH may exacerbate negative responses in organisms, or increase their susceptibility to either or both oxygen and pH.
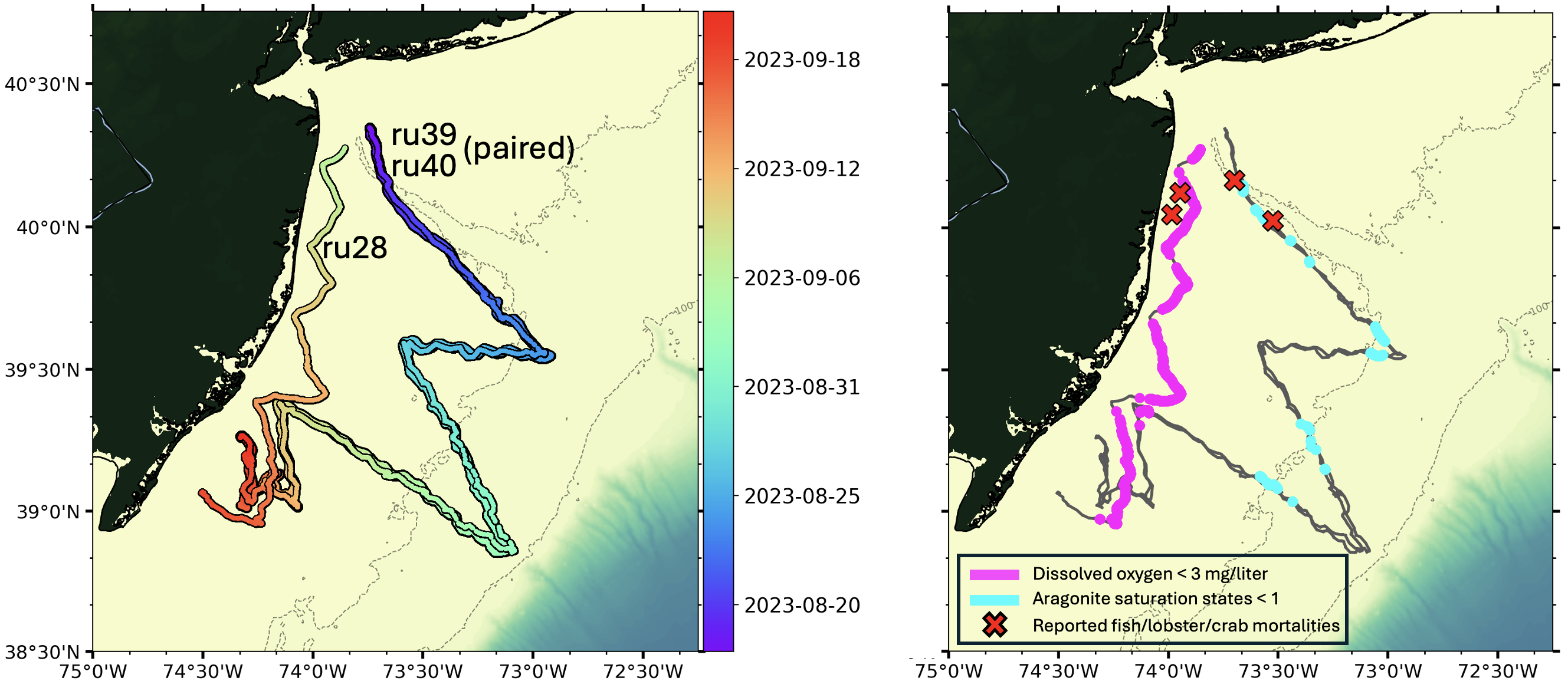
Left: Mission tracks of three gliders (ru28, ru39, ru40) deployed off the coast of New Jersey in August and September. Gliders ru39 and ru40 were deployed as a pair along the same mission track. All gliders had sensors measuring temperature and salinity. Gliders ru28 and ru40 each had an additional sensor measuring dissolved oxygen (no pH or aragonite saturation state), and glider ru39 had an additional sensor measuring pH (no dissolved oxygen).
Right: Locations of hypoxic levels of dissolved oxygen (magenta; < 3 mg/liter) and low aragonite saturation state (cyan; < 1) measured along the glider mission tracks and locations of reported fish, lobster, and/or crab mortalities (red X).
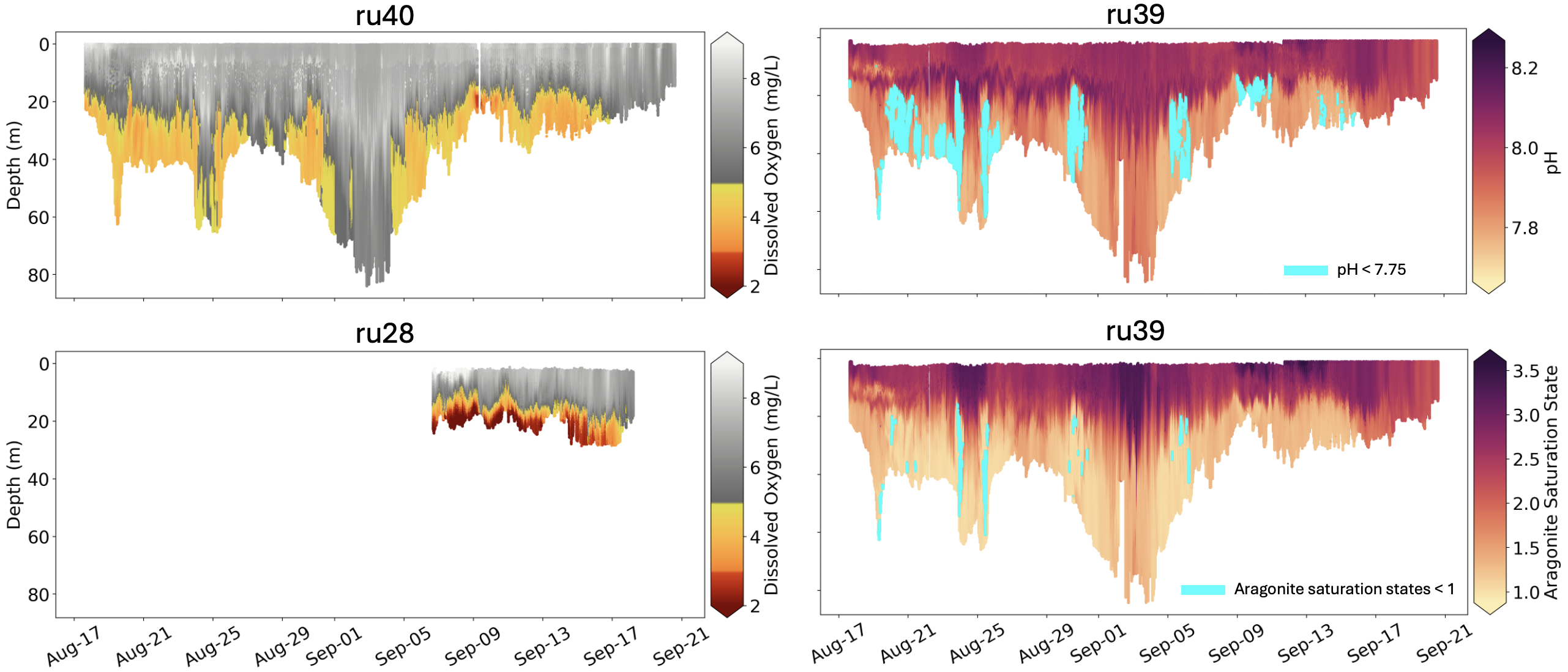
Complete cross-sections of dissolved oxygen concentrations (left top and bottom), pH (right top), and aragonite saturation state (right bottom) measured along the mission tracks during the deployments of the three gliders (ru28, ru39, ru40) during August and September. Dissolved oxygen concentrations between 3-5 mg/liters are expressed as orange & yellow, and hypoxic concentrations < 3 mg/liter are expressed as red. pH values < 7.75 and aragonite saturation states < 1 are highlighted in cyan.
Reports of fish, lobster, and crab mortalities
During the time when low dissolved oxygen and pH were observed, numerous mortalities of fish, lobsters and crabs within the sampling area were reported. The mortalities were observed in bottom waters primarily off the coast of Monmouth and Ocean Counties and included the Mud Hole, as far east as Lillian wreck, and southward in Sea Girt and Axel Carlson Reefs and the surrounding areas. Mortalities were reported for American lobsters, Jonah crab, Atlantic rock crab, spider crabs, black sea bass, and tautog were reported not only in pots where trapped organisms would not have been able to escape poor conditions, but also on the open bottom. This observation suggests that if low dissolved oxygen and/or pH were indeed the culprit for these reported mortalities, the area may have been extensive enough that they could not escape in time.
Mortalities associated with low dissolved oxygen in New Jersey coastal waters or other locations is not new. The most extreme hypoxic event documented in the State occurred during the summer of 1976, whereby mass mortalities of marine organisms occurred over a 12,000 km2 area. More recently, numerous fish, lobster, and crab mortalities were associated with low dissolved oxygen in Cape Cod Bay in September 2019, and hypoxic conditions have been identified as the culprit of mortalities and changes in bottom water communities in the Gulf of Mexico’s notorious summer-time ‘Dead Zone’ since the beginning of annual observations that started in 1985.
What causes low dissolved oxygen and pH in bottom water?
A common seasonal phenomenon in New Jersey coastal shelf waters is strong summer stratification whereby cold water near the bottom is capped off by warm, highly oxygenated water near the surface. This creates a two-layer stacking of water off the NJ coast with little exchange of oxygen between them. Furthermore, nutrient inputs from rivers and estuaries support the growth of single-celled plants called phytoplankton in the surface layer, which produces surface layer oxygen through the process of photosynthesis. However, these surface plants eventually die and sink to the bottom layer where bacteria break them down. As bacteria metabolize, they remove oxygen from the bottom layer and produce carbon dioxide.
Without the periodic input of surface water oxygen, the bottom layer can reach much lower dissolved oxygen levels that can lead to hypoxia (low dissolved oxygen) and acidification (low pH) in bottom waters. These seasonal patterns of biologically-driven seawater pH change are occurring simultaneously with ongoing global ocean acidification caused by the ocean’s absorption of the increasing concentrations of atmospheric carbon dioxide since the beginning of the Industrial Revolution. Globally, ocean acidity has increased by about 28% since the 1800s (equivalent to a decrease of 0.1 pH units, or about -0.015 pH units per decade). Therefore, local coastal processes can rapidly exacerbate the long-term rate of ocean acidification already occurring in the region.
Is this year different from previous years?
Because a number of drivers can cause low bottom-water dissolved oxygen and pH, it is often difficult to pinpoint specifically what causes lower values or more spatially widespread events in one year compared to others. Data from NJDEP (1979-2005) showed dissolved oxygen concentrations less than 5 mg/liter occurred every year during the summer season, and some years and locations experienced concentrations < 2 mg/liter. However, the co-occurrence of reported fish, lobster, and crab mortalities, the observed values of pH and aragonite saturation state that were lower than previously measured for recent summers in this region, and the combination of lower dissolved oxygen and low pH and aragonite saturation state, point to the fact that the poor conditions observed this summer could have been more pronounced than in previous years.
The years in which lower than average dissolved oxygen concentrations and pH occur or where lower bottom water dissolved oxygen and pH are more spatially widespread could be due to a number of factors, including earlier onset of or stronger seasonal stratification that limit oxygen exchange between surface and bottom, increased inputs of nutrients that act to increase the production of phytoplankton and/or the spatial area of phytoplankton growth, environmental conditions (temperature, nutrients, predator-prey dynamics) that act to facilitate the growth of certain phytoplankton species (e.g., harmful algal blooms) that are not readily eaten and removed, and increased ocean temperatures that increase metabolism, including bacterial metabolism that will reduce oxygen and pH in bottom waters.
What are the next steps in your research?
Faculty, students and staff at Rutgers are continuing to analyze the data collected to identify the specific causes of this summer’s low dissolved oxygen and pH event. Historical analysis of summer-time dynamics related to low bottom water dissolved oxygen are also underway. Events such as these that may prevent the ability to sustain normal populations of marine organisms are concerning, not only for the ocean ecosystem but also for the local economy and commercial and recreational fishing industries. Understanding the factors that cause these events will aid in projecting the severity and duration of these events under ongoing climate change and provide important support for guiding the State’s policy options and identifying priorities for science and monitoring.
If you would like to report any organism mortalities observed over this past summer, email associate professor Grace Saba at saba@marine.rutgers.edu.
The RUCOOL Operations Center maintains the world’s most advanced coastal ocean observatory using a breadth of platforms consisting of satellite imagery, a multi-static HF radar network for surface current mapping and waves, and a fleet of long-duration autonomous underwater vehicles (gliders) equipped with physical, chemical and biological sensors.
Support for this work was provided by grants from the New Jersey Research and Monitoring Initiative and New Jersey Department of Environmental Protection’s Bureau of Marine Water Monitoring.
Original article at Rutgers NJAES Newsroom

